
Positive and negative charges attract each other, so stationary electrons would fall into the positive nucleus. Rutherford’s model required that the electrons be in motion. Rutherford’s model placed the other type of charge-the negative electrons-in orbit around this nucleus. When a positively charged alpha particle strikes a nucleus, it reverses direction, much as a cue ball reverses direction when it strikes another billiard ball. The only way to account for the particles that reversed direction when they hit the gold foil was to assume that nearly all of the mass, as well as all of the positive charge in each individual gold atom, is concentrated in a tiny center or nucleus. Note that this drawing is not to scale the electron orbits are much larger relative to the size of the nucleus. (b) From this experiment, he concluded that the atom must be constructed like a miniature solar system, with the positive charge concentrated in the nucleus and the negative charge orbiting in the large volume around the nucleus. Rutherford’s Experiment: (a) When Rutherford allowed α particles from a radioactive source to strike a target of gold foil, he found that, although most of them went straight through, some rebounded back in the direction from which they came. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you."įigure 1. Rutherford wrote, "It was quite the most incredible event that has ever happened to me in my life. About 1 in 8000 of the alpha particles, however, completely reversed direction and bounced backward from the foil. Most of these particles passed though the gold foil just as if it and the atoms in it were nearly empty space. Alpha particles (α particles) are helium atoms that have lost their electrons and thus are positively charged. He bombarded an extremely thin piece of gold foil, only about 400 atoms thick, with a beam of alpha particles (Figure 1). In 1911, British physicist Ernest Rutherford devised an experiment that provided part of the answer to this question. The next step was to determine where in the atom the positive and negative charges are located. (It is the flow of these particles that produces currents of electricity, whether in lightning bolts or in the wires leading to your lamp.) Because an atom in its normal state is electrically neutral, each electron in an atom must be balanced by the same amount of positive charge. Named the electron, this particle is negatively charged. The first of these smaller particles was discovered by British physicist James (J. Instead, the atom is a complex structure composed of still smaller particles.
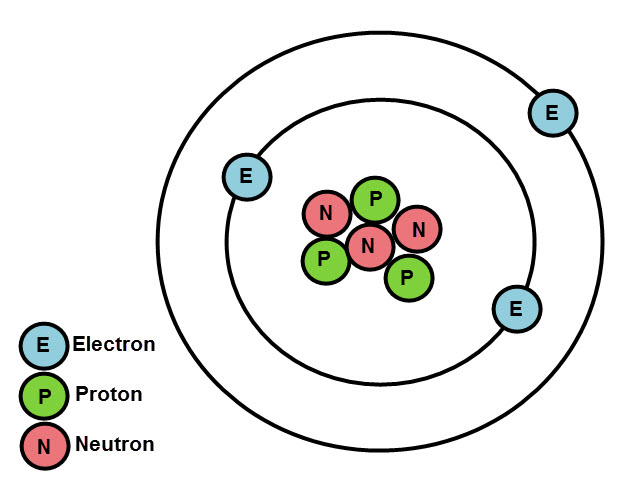
It took until the twentieth century, however, for scientists to invent instruments that permitted them to probe inside an atom and find that it is not, as had been thought, hard and indivisible.

The idea that matter is composed of tiny particles called atoms is at least 25 centuries old. Explain the behavior of electrons within atoms and how electrons interact with light to move among energy levels.

Describe the structure of atoms and the components of nuclei.By the end of this section, you will be able to:


 0 kommentar(er)
0 kommentar(er)
